Wunderbar! A Hydrogen Based Perpetual Motion Machine. [View all]
I came across this paper in the primary scientific literature: High-Stability ZnAl2O4 Spinel-Supported Nickel Catalyst for High-Temperature Syngas Methanation Gengrui Zhang, Yan Li, Yong Chen, Xinning Hao, Xianhua Zhang, Shuai Wang, Jingdong Lin, Yong Wang, and Shaolong Wan Industrial & Engineering Chemistry Research 2023 62 (41), 16668-16675.
We hear, regrettably, about "green hydrogen," which more or less doesn't exist on any scale that matters. The hydrogen salespeople and salesbots who come to DU to sell fossil fuels by rebranding them as "hydrogen" are selling something worse than snake oil, something worse than "health cigarettes."
A Giant Climate Lie: When they're selling hydrogen, what they're really selling is fossil fuels.
Hydrogen is not primary energy and as such its manufacture destroys exergy meaning that one uses more fossil fuels to make the intermediate hydrogen than one would have used to have combusted the dangerous fossil fuels directly. Almost all of the hydrogen on this planet is made by steam reforming (at high temperatures) of fossil fuels:
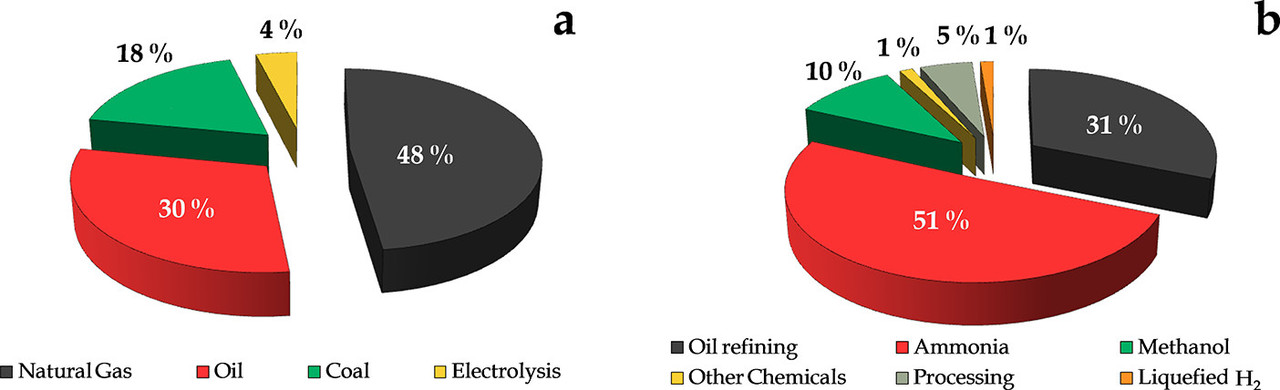
The caption:
Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review Laura Santamaria, Gartzen Lopez, Enara Fernandez, Maria Cortazar, Aitor Arregi, Martin Olazar, and Javier Bilbao, Energy & Fuels 2021 35 (21), 17051-17084]
And now we have this paper that tells us we can make synthetic dangerous fossil fuels, in this case methane, from, um, hydrogen.
To meet this formidable challenge, supported catalysts using varied metals such as Rh, Ru, Ni, Co, etc. have been intensively explored, (6−11) and so are the effects of varied supports including Al2O3, ZrO2, TiO2, SiO2, etc. (12−18) Among the large number of catalysts investigated for the reaction, nickel-based catalysts have been recognized as the most appropriate ones for methanation due to their high catalytic activity, high selectivity to methane, and relatively low price. However, it is known that conventional nickel-based catalysts can deactivate rapidly because of the sintering of Ni particles (during the reduction pretreatment and/or reaction) and carbon deposition. To improve the activity and stability of the nickel/alumina systems, lots of studies have been conducted to optimize the composition, the preparation method, and the catalyst support, (19−23) as well as the addition of promoters such as Ce, Zr, La, etc. (24−29) However, there are still limited reports on the complete syngas methanation over Ni-based catalysts at temperatures above 450 °C, probably due to the formidable challenge confronted in this process. Gao et al. investigated the effect of the structures and surface properties of Al2O3 supports calcined at different temperatures and found that the Ni catalysts supported on α-type Al2O3 obtained by calcining the commercial γ-Al2O3 at 1200 °C are most active and thermally stable in CO methanation. (30) However, the surface area of the obtained support is very small (only 9.4 m2/g), and notable aggregation of Ni particles occurred after the hydrothermal treatment (particle size increased from 17 to 31 nm), which led to a partial loss in activity. Liu et al. investigated the Ni–Mg/Al2O3 catalysts prepared with different methods and revealed that dispersed NiO interacting with MgO and the support would prevent the agglomeration of Ni crystallites and was likely to improve the activity and stability of the catalyst at high temperatures (up to 700 °C) for methanation. (23) Nonetheless, the major species of the support that reacts with dispersed NiO remained unclear.
The scarce successful examples in the literature thus reveal a need to develop a useful method to prepare highly efficient nickel catalysts for high-temperature syngas methanation. In previous work, we synthesized supported PdZn catalysts using a ZnAl2O4 spinel as a support to provide atomic-level control of the zinc source for the formation of PdZn alloy, thanks to the unique spinel structure and the strong interaction between the metal and the support. The advanced Pd/ZnAl2O4 catalysts show superior activity and stability toward Syngas conversion and methanol-steam-reforming reaction, even upon ppm Pd loading. (31,32) Herein, we came up with a novel design to synthesize a ZnAl2O4-supported/dispersed Ni metal catalyst, where the zinc aluminate spinel featuring superior thermal and hydrothermal stability is employed to support and disperse the active Ni component. The obtained Ni/ZnAl2O4 demonstrated comparable reactivity but better stability during the methanation test conducted above 450 °C, compared with the Ni/Al2O3 counterparts. Furthermore, after high-temperature steam treatment, 35% Ni/ZnAl2O4 still maintains high activity, while 35% Ni/Al2O3 no longer has activity. The strong interaction between Ni and ZnAl2O4 can not only suppress the sintering of Ni particles but, more importantly, prevent the formation of significant NiAl2O4 that causes permanent activity loss during the methanation process...
Actually, I'm fairly sure the authors of this paper are well aware that perpetual motion machines don't work. This is a scheme to make dangerous natural gas from coal, the overwhelming, by far, source of hydrogen in China. The environmental costs of this scheme are, of course, appalling, but don't kid yourself, it could go industrial.
This scheme is every bit as bad as the idea of using hydrogen as a consumer fuel. It is true however, that methane's physical properties, although appalling, are superior to those of hydrogen itself, as the critical temperature of methane is 190 Kelvin, whereas that of hydrogen is 33 Kelvin.
We live in a time where scientific illiteracy is so high that people actually believe that anything, including perpetual motion machines, can be excused by reference to hydrogen.
Hydrogen is an exceedingly dirty fuel since making it wastes primary energy, most of which on this planet, is still generated using dangerous fossil fuels.
Have a nice Saturday evening.