Environment & Energy
Related: About this forumAdventures in "Green" Batteries, Fluorinated Ionic Liquids (and other PFAS) in European Waters.
The paper to which I'll briefly refer in this post is this one:
Nontarget and Suspect Screening of Fluorinated Ionic Liquids and PFAS in European Wastewaters Using Supercritical Fluid Chromatography Selina Tisler, Jonathan Zweigle, Maria Kregler Gotil, Saskia Finckh, Werner Brack, Eva-Maria Braxmaier, Corina Meyer, Juliane Hollender, Tina Kosjek, Emma L. Schymanski, Pontus Larsson, Anna Kärrman, Erica Selin, Dalia Elabbadi, Harry Elliss, Barbara Kasprzyk-Hordern, Tim Boogaerts, Adrian Covaci, Herbert Oberacher, Harold Flores Quintana, Foon Yin Lai, Lutz Ahrens, Azziz Assoumani, Frederic Béen, and Jan H. Christensen Environmental Science & Technology 2025 59 (39), 21300-21311
The full paper is open access, so there isn't a need to discuss it in any tremendous depth; interested persons can read it themselves independently of my often acerbic commentary.
The paper caught my eye for several reasons, one of which is my long standing interest in ionic liquids, a series of what are effectively salts with low melting points. They are said to be "green" because they exhibit low volatility. I have had occasion to have dinner with a major contributor to this science; one of the high points of my recent intellectual life. Another reason is my interest in the underutilized supercritical fluid chromatography, which displaces organic solvents like acetonitrile and methanol with easily treated carbon dioxide in its readily accessible supercritical state, in which it is a fluid that cannot be described as either a liquid or a gas. Finally, I am always interested in mass spectrometry and its application for environmental purposes, although my professional interest has nothing to do with environmental issues.
To be perfectly honest, I wasn't aware that one of the applications of ionic liquids - which I have regarded in a more academic sense - is in batteries, although I was aware of hexfluorophosphate in them somehow without connecting it to the idea of ionic liquids.
Anyway, from the paper's introduction:
In addition to well-characterized PFAS, other fluorinated compounds are gaining increased attention. Many pharmaceuticals and pesticides fall under the Organisation for Economic Co-operation and Development (OECD) PFAS definition─organic substances containing at least one fully fluorinated carbon atom (e.g., CF3 groups). (14) In this study, compounds are defined as low-fluorinated if their molecular fluorine mass percentage is below 40%, a threshold commonly associated with fluorinated pharmaceuticals and pesticides (Figure S1). (15) Many of these low-fluorinated compounds can act as precursors to trifluoroacetic acid (TFA), (16,17) and significantly contribute to the total fluorine load in wastewater and sludge, (15) as they can occur at concentrations ten to hundred times higher than conventional PFAS. (18)
Recently, fluorinated ionic liquids have emerged as concerning environmental pollutants. (19) Their diverse industrial applications, particularly in lithium-ion batteries for electric vehicles, are rapidly increasing alongside the increasing global demand for energy storage solutions. (20) Despite their rising production, knowledge regarding their environmental fate remains limited. Recent studies have reported widespread occurrence of ionic liquids in the environment, such as bis(trifluoromethylsulfonyl)imide (NTf2–), which has been detected in 46% of tap water samples across multiple countries, (21) and hexafluorophosphate (PF6–) has been found ubiquitously in German river waters. (22) Ionic liquids are suspected to account for a substantial fraction of the environmentally persistent “PFAS dark matter”. (20,23)
Two figures from the text:
Workflows:
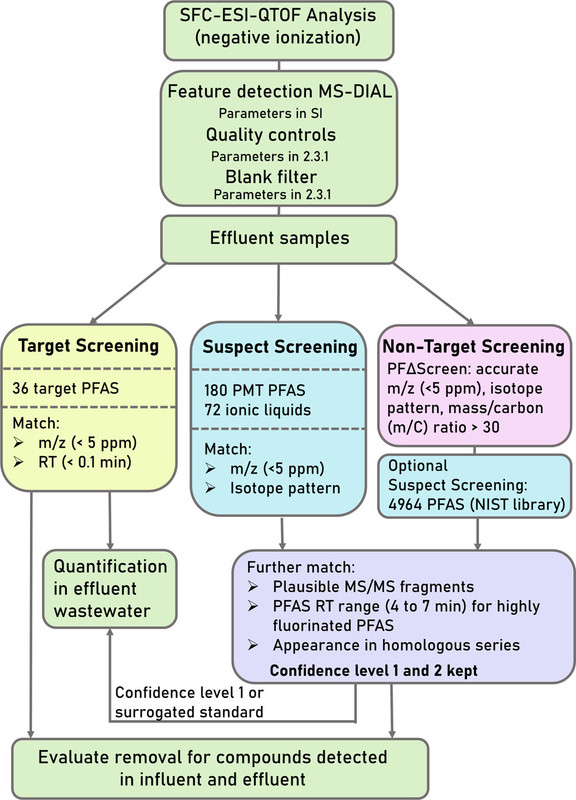
The caption:
Structures of some "substitute" PFAS and ionic liquid counterions:
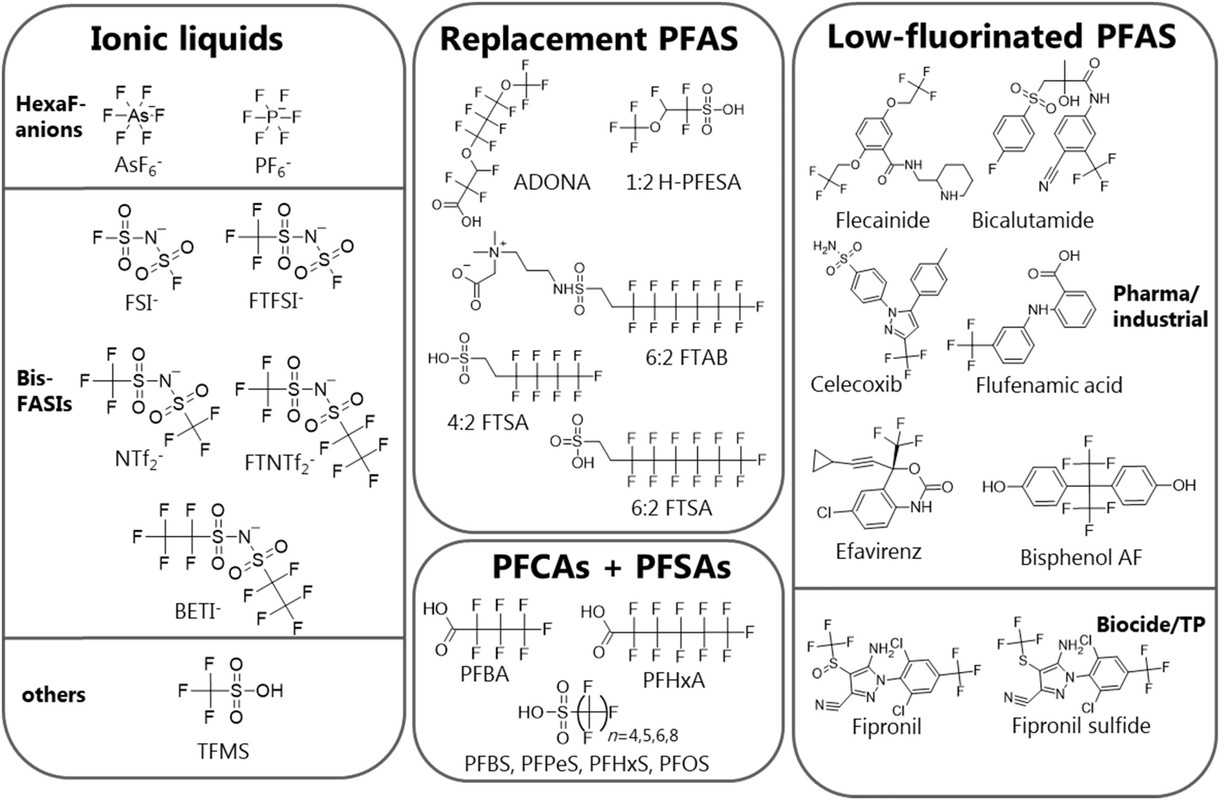
The issue that arises is a question whether a "substitute" is actually worse than the problematic compound it is designed to replace.
This effect takes place not only in PFAS but in many other areas as well.
Indeed, a battery is a device that destroys exergy - the energy which is useful to perform a task. Seen this way, one should expect that the tiresome claim that batteries are somehow green has always been questionable. Chemical pollution only makes things worse.
From the article's conclusion:
Again, the full article is available in open access. Anyone can read it.
Have a nice weekend.