Environment & Energy
Related: About this forumFluorine Flows in China.
The paper I'll discuss in this post is this one: Advancing Sustainable Fluorine Management in China Based on Evolution of the Anthropogenic Cycle during 2000–2020 Kun Hu, Hui Gong, Guoning Zhou, Chao Zhang, Shuyan Zhou, Guangming Li, Ling Chen, and Xiaohu Dai Environmental Science & Technology 2025 59 (11), 5521-5533.
The chemistry of fluorine represents one of the most important environmental issues in the world, impacting health and technology in the form of PFOS (perfluoro organic substances), extreme global heating (in the form of gaseous fluorocarbons, chlorofluorocarbons, and sulfur fluorides), and in a case near to my heart, nuclear energy technology.
The world industrial powerhouse after (and actually preceding) the fall of the United States, is China, and thus fluorine flows in China are important to all humanity.
From the text of the paper under discussion:
Sustainable F resource management necessitates balancing extraction, utilization, recycling, and environmental safeguards to meet the present needs without compromising future availability. As the global leader in F production and consumption (≥55% of fluorochemicals output), (21) China faces acute supply risks. While industry consolidation policies, (22−24) the inclusion of strategic minerals list, (5) export tariffs, (25−27) and capacity controls for traditional sectors and fluorinated commodities (e.g., hydrofluoric acid (HF)) (28) stabilized short-term supply in the past two decades, the impending scarcity is still signaled by low static reserves-to-production ratio (R/P ratio, representing the number of years the country or region has left to mine at the current level of ore production, expressed in years, which changes dynamically each year). (29,30) China’s static fluorspar R/P ratio was less than 8 years in 2020 according to statistics from the United States Geological Survey (USGS). (31) Given the nation’s pivotal role in global fluorochemicals production, such depletion would destabilize international supply chains for F-dependent technologies, including electronics, energy storage, and agrochemicals. This disruption could also propagate cascading effects across industries reliant on fluorinated materials, threatening manufacturing stability worldwide. Concurrently, rapid growth in emerging sectors (e.g., photovoltaics and lithium batteries) exacerbates demand–supply mismatches, (32) compounded by fragmented regulations: (33−35) strict discharge limits for legacy industries (36−39) contrast with inadequate controls for newer applications (e.g., semiconductor etching), resulting in inefficient recycling and persistent pollution. (40)
A systematic understanding of China’s F resource lifecycle─tracking extraction, transformation, utilization, and waste management across sectors─is critical for advancing sustainable governance. Material flow analysis (MFA) provides a robust framework to map these dynamics, quantifying the spatial, temporal, and sectoral distribution of F resources. Such analysis enables policymakers to identify inefficiencies, optimize recycling, and mitigate environmental risks. (41) However, previous MFA studies remain fragmented, focusing narrowly on specific compounds (e.g., fluorocarbons (42−44) and perfluorinated compounds (45−48)) or sectors (e.g., metallurgy (49) and electrolytic aluminum (50,51)), while neglecting the diversity of F applications. For instance, F-containing materials serve distinct roles across industries: as functional additives (e.g., pharmaceutical intermediates), embedded components (e.g., fluorocarbon refrigerants), or single-use consumables (e.g., semiconductor etching agents). Each application follows unique flow pathways with varying environmental consequences. Existing frameworks also fail to address evolving sectoral dynamics, particularly post-2010 innovations in F-based green technologies...
Bold, italics and underlining are all mine. Anyone familiar with my writings, by the way, will understand that I question what the much abused word "green" might actually mean, and I insist it's more familiar in abuse than meaningful use.
It's useful to look at the pictures in this paper. First Sankey diagrams of Chinese fluorine flows in recent times:
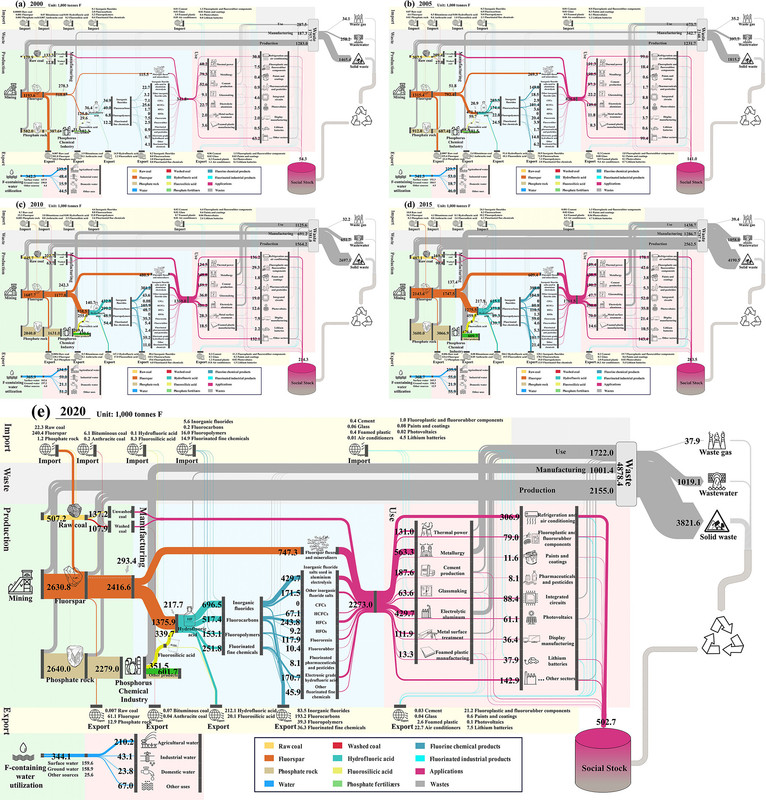
The caption:
Some additional text:
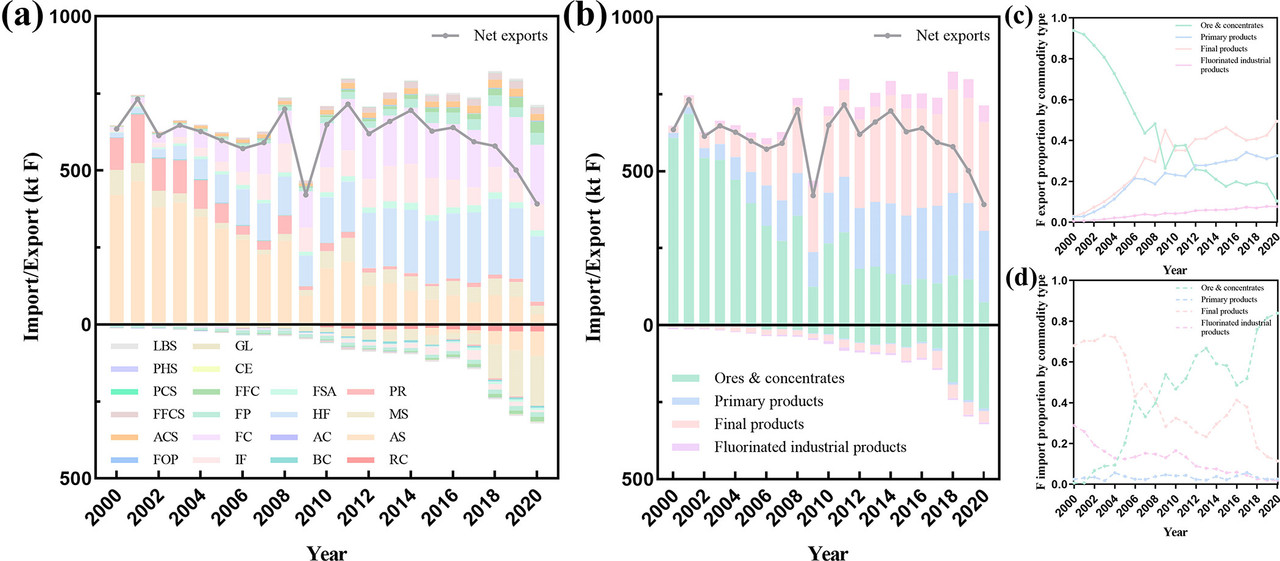
Another figure from the text:
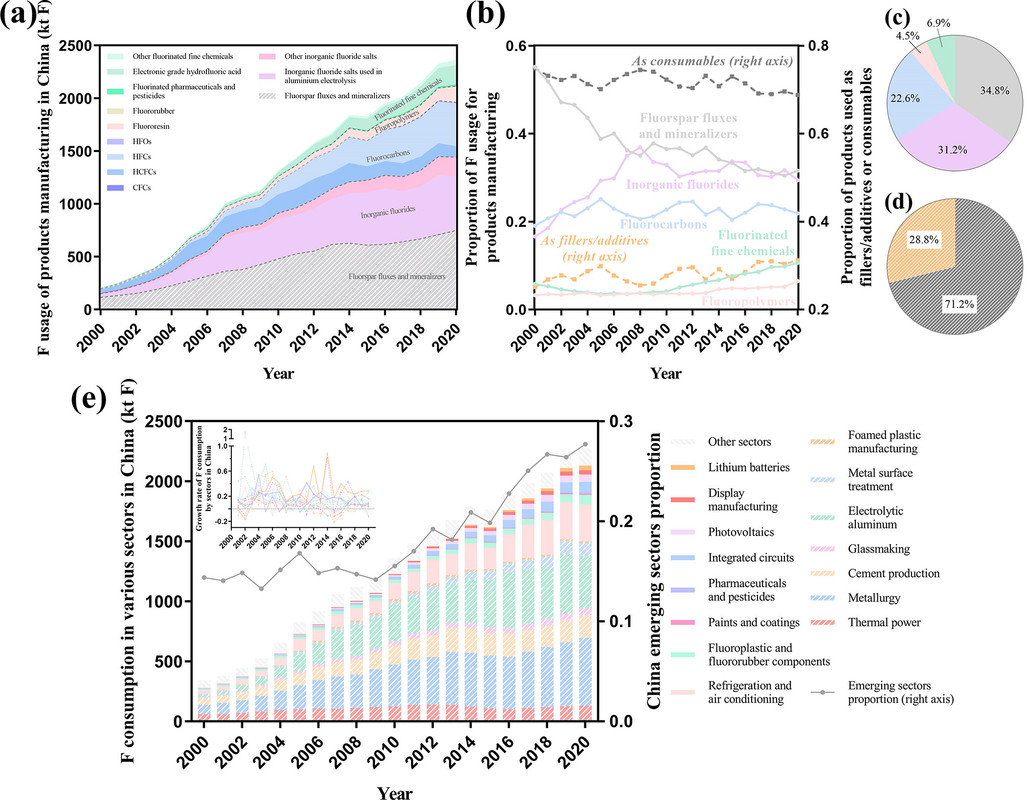
The caption:
Two of these applications, lithium batteries and photovoltaics, are often represented as "green," a description with which I emphatically disagree, considering such use as abuse, since materials requirements make them unsustainable, fluorine requirements being only a part of the problem, the mere tip of the tailings pile, since the word "iceberg" is now threatened by extreme global heating, which neither photovoltaics nor batteries have done anything to address, faith in them having actually made things worse.
The carbon fluorine bond is one of the strongest in chemistry. Breaking it photochemically - really the best option in my view - requires high energy radiation, short wave length UV, x-rays or gamma radiation. These are accessible from components of used nuclear fuel, an application I am working to push to my son and anyone else who will listen, primarily from radioisotopes of cesium, in particular 134Cs and 137Cs, the latter an isotope that generates some huge, but silly, fear.
I've advanced some continuous flow ideas to my son on this topic. It cannot be said, however, under the best of circumstances, that the recovery of fluorine from atmospheric and water pollutants will necessarily lead to a closed system. I very much doubt it will.
For nuclear fuel reprocessing I favor fluoride volatility methods, but the high energy density of nuclear fuel makes such use relatively trivial. I oppose nuclear fuel enrichment, regarding it is as unnecessary and perhaps unwise, particularly since many nations, fearful of the criminal Putin-Trump axis are considering developing nuclear armaments. This, of course, is not a good thing. We need to isotopically denature weapons grade uranium and plutonium, not produce more of it.